This text is published under creative commons licensing, for referencing and adaptation, please click here.
Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds represent extreme cases that are seldom encountered in any but very simple substances, a brief discussion of these two extremes helps explain why substances with different kinds of chemical bonds have very different properties. Ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist of molecules, which are groups of atoms in which one or more pairs of electrons are shared between bonded atoms. In a covalent bond, atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share. This chapter will focus on the properties of covalent compounds.
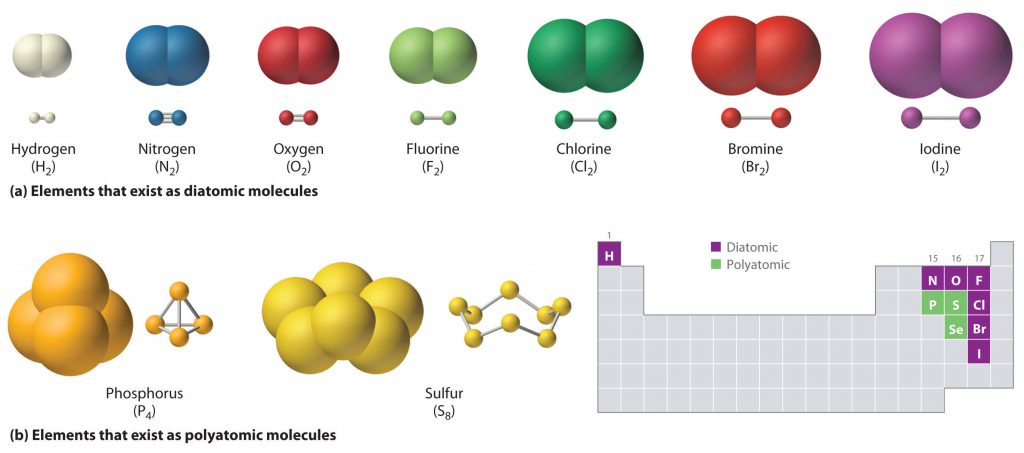
Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the fundamental chemical properties of a covalent compound. Thus, the term molecular compound is used to describe elements that are covalently bonded and to distinguish the compounds from ionic compounds. Some pure elements exist as covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic (“two atoms”) molecules H2, N2, O2, F2, Cl2, Br2, and I2 (part (a) in Figure 4.1 ). Similarly, a few pure elements exist as polyatomic (“many atoms”) molecules, such as elemental phosphorus and sulfur, which occur as P4 and S8 (part (b) in Figure 4.1 ).

Figure 4.1 Elements That Exist as Covalent Molecules. (a) Several elements naturally exist as diatomic molecules, in which two atoms (E) are joined by one or more covalent bonds to form a molecule with the general formula E2. (b) A few elements naturally exist as polyatomic molecules, which contain more than two atoms. For example, phosphorus exists as P4 tetrahedra—regular polyhedra with four triangular sides—with a phosphorus atom at each vertex. Elemental sulfur consists of a puckered ring of eight sulfur atoms connected by single bonds. Selenium is not shown due to the complexity of its structure.
Each covalent compound is represented by a molecular formula, which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule. The subscript is written only if the number of atoms is greater than 1. For example, water, with two hydrogen atoms and one oxygen atom per molecule, is written as H 2 O. Similarly, carbon dioxide, which contains one carbon atom and two oxygen atoms in each molecule, is written as C O 2 .

Covalent compounds that predominantly contain carbon and hydrogen are called organic compounds. The convention for representing the formulas of organic compounds is to write carbon first, followed by hydrogen and then any other elements in alphabetical order (e.g., CH4O is methyl alcohol, a fuel). Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds; they include both covalent and ionic compounds. The convention for writing inorganic compounds, involves listing the component elements beginning with the one farthest to the left in the periodic table, as in CO2 or SF6. Those in the same group are listed beginning with the lower element and working up, as in ClF. By convention, however, when an inorganic compound contains both hydrogen and an element from groups 13–15, hydrogen is usually listed last in the formula. Examples are ammonia (NH3) and silane (SiH4). Compounds such as water, whose compositions were established long before this convention was adopted, are always written with hydrogen first: Water is always written as H2O, not OH2. Typically this distinguishes when hydrogen is participating in a covalent bond rather than an ionic interaction, as seen in many of the inorganic acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), as described in chapter 3 .
In Chapter 3, we saw that ionic compounds are composed predominantly of a metal + a nonmetal. Covalent molecules, on the otherhand, are typically composed of two nonmetals or a nonmetal and a metalloid. This is an initial screening method that you can use to categorize compounds into the ionic or the covalent cagetogy.
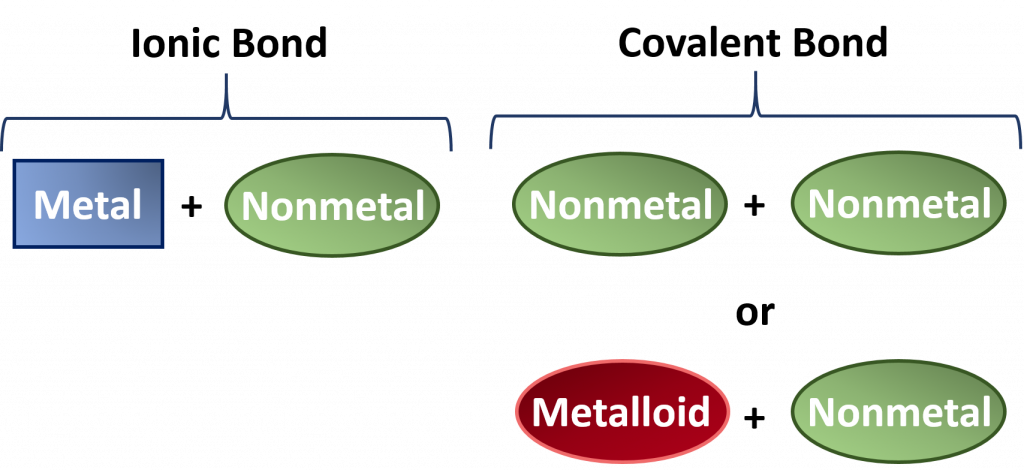
Figure 4.2 Recognizing Ionic vs Covalent Compounds. Typically compounds that are formed from a combination of a metal with a nonmetal have more ionic bond character whereas compounds formed from two nonmetals or a metalloid and a nonmetal show more covalent character. Although compounds usually lie on a spectrum somewhere between fully ionic and fully covalent character, for naming purposes, this guideline works well.
Chapter 3 described how electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell following the octet rule . However, there is another way an atom can achieve a full valence shell: atoms can share electrons to reach the octet state (or the duet state in the case of hydrogen).
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) We can represent the two individual hydrogen atoms as follows:
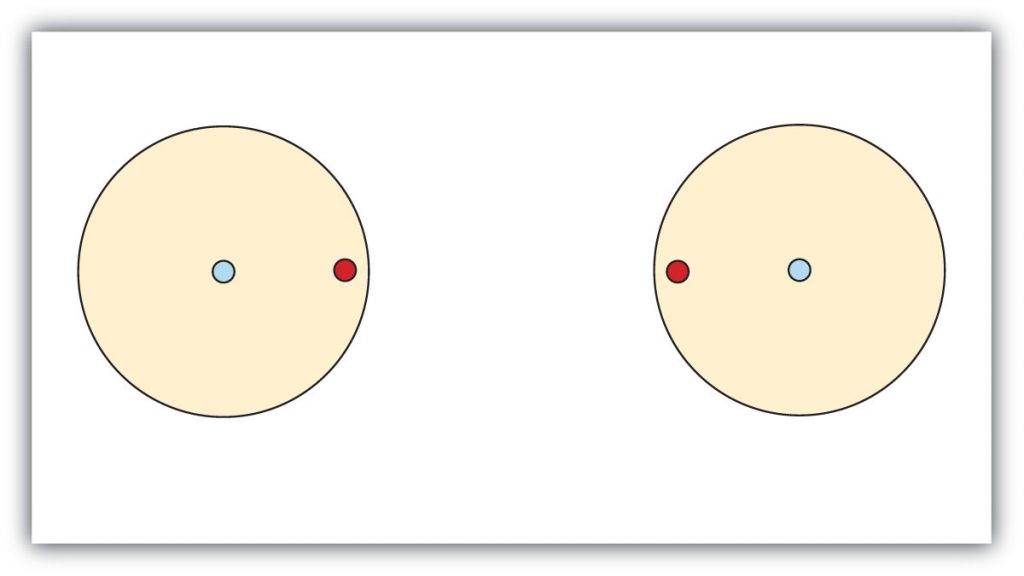
In this situation neither hydrogen can reach the preferred duet state. In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows:
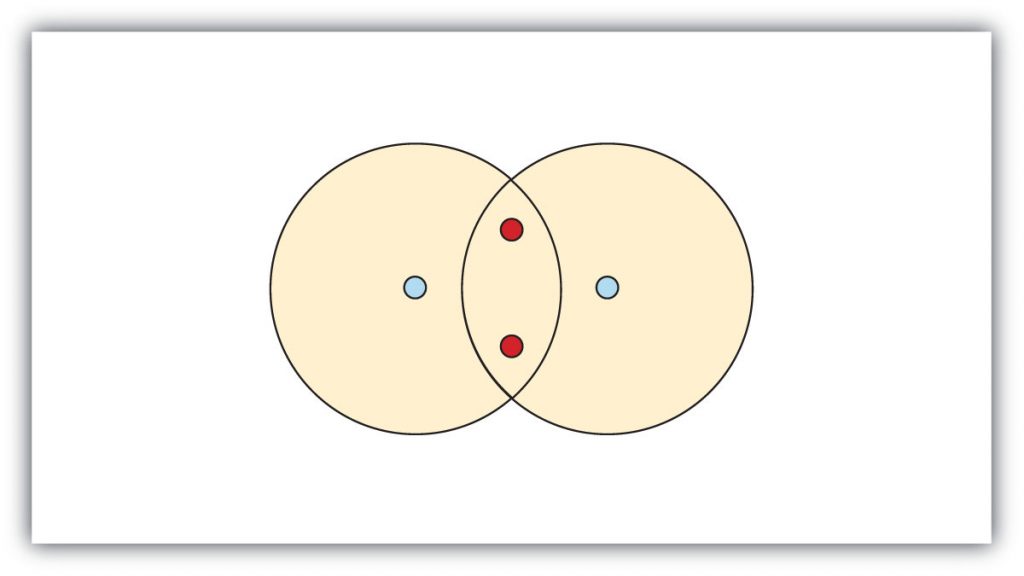
By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells. Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. In this configuration, each hydrogen has an electron configuration equivalent to that of the noble gas, helium. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound. For example, one molecule of water would contain two hydrogen atoms and one oxygen atom (H2O).
Chemists frequently use Lewis electron dot diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows:
The Lewis diagram of two hydrogen atoms sharing electrons looks like this:
This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
Remember that the dash, also referred to as a single bond, represents a pair of bonding electrons.
The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; 1 pm = 1 × 10 −12 m). This particular bond length represents a balance between several forces: (1) the attractions between oppositely charged electrons and nuclei, (2) the repulsion between two negatively charged electrons, and (3) the repulsion between two positively charged nuclei. If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles.
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Two separate fluorine atoms have the following electron dot diagrams:
Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule:
The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons:
Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. Consider a molecule composed of one hydrogen atom and one fluorine atom:
Each atom needs one additional electron to complete its valence shell. By each contributing one electron, they make the following molecule:
In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The circles show how the valence electron shells are filled for both atoms (recall that hydrogen is filled with two electrons).
Larger molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. For example, water, with two hydrogen atoms and one oxygen atom, and methane (CH4), with one carbon atom and four hydrogen atoms, can be represented as follows:
Atoms typically form a characteristic number of covalent bonds in compounds. Figure 4.3 shows valence electron configurations of each element family (or column).
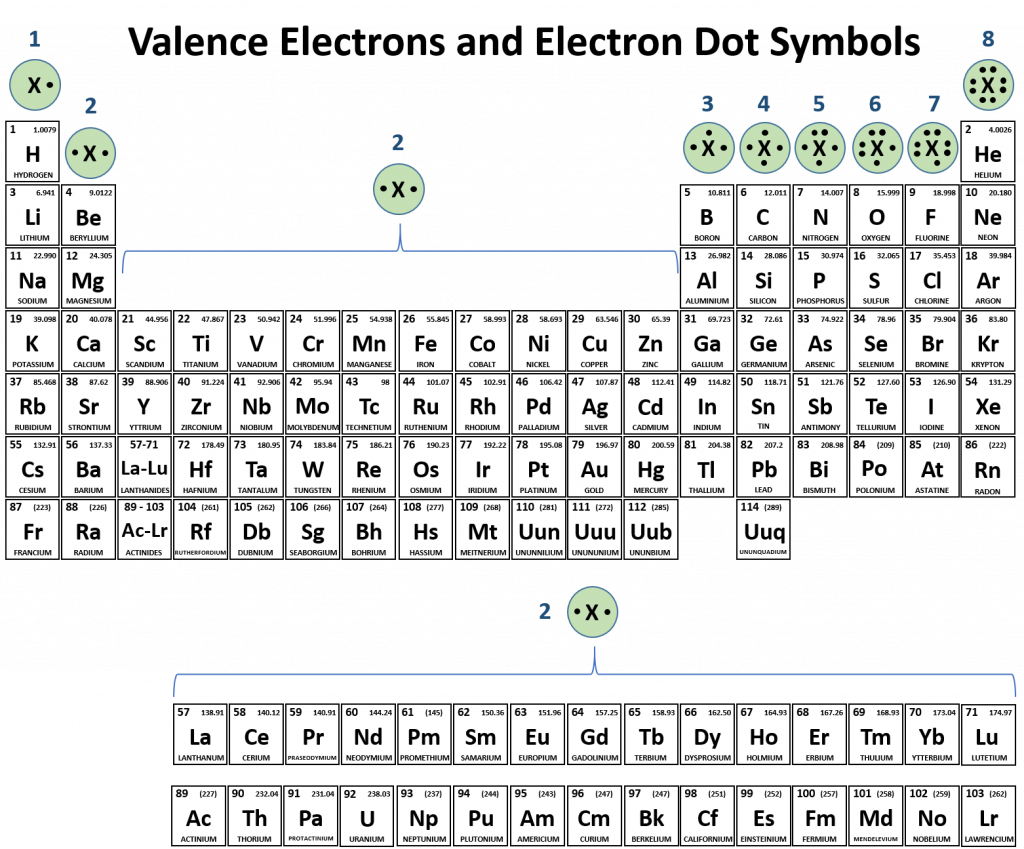
Fig 4.3 Periodic Table with Lewis Structures. Each family shows a representative lewis structure for that group of elements. For the nonmetals (Families 4A, 5A, 6A, and 7A) they can accept a complementary number of shared bonds to reach the octet state. Family 4A can share 4 covalent bonds (4 + 4 = 8), whereas Families 5A, 6A, and 7A can share 3, 2, and 1 covalent bond(s), respectively, to achieve the octet state. Exceptions to the octet rule do exist. For example, hydrogen can be considered to be in Group 1 or Group 7A because it has properties similar to both groups. Hydrogen can participate in either ionic or covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. As it has one electron to start with, it can only make one covalent bond. Similarly, boron has 3 electrons in its outer shell. This nonmetal typically forms 3 covalent bonds, having a maximum of 6 electrons in its outer shell. Thus, boron can never reach the octet state. Other atoms can have expanded orbitals and accept additional covalent bonds. Two of these that are important for living systems are sulfur and phosphorus. By the octet rule, sulfur can make 2 covalent bonds and phosphorus 3 covalent bonds. Sulfur can also have expanded orbitals to accept 4 or 6 covalent bonds, and phosphorus can expand to 5 covalent bonds.

In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares only two electrons. Consider carbon dioxide (CO2). If each oxygen atom shares one electron with the carbon atom, we get the following:
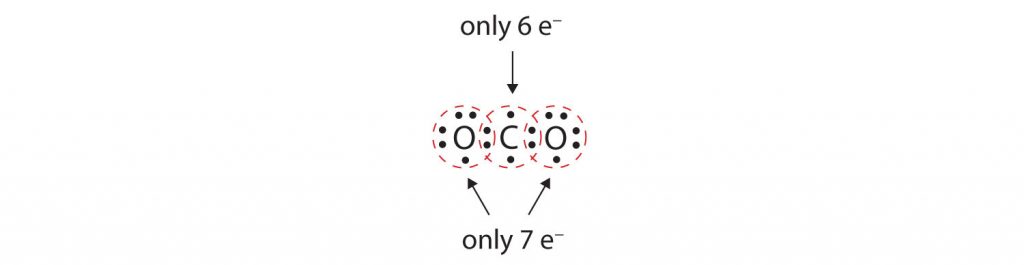
This does not give either the carbon or oxygen atoms a complete octet; The carbon atom only has six electrons in its valence shell and each oxygen atom only has seven electrons in its valence shell. Thus, none of the atoms can reach the octet state in the current configuration. As written, this would be an unstable molecular conformation.
Sometimes more than one pair of electrons must be shared between two atoms for both atoms to have an octet. In carbon dioxide, a second electron from each oxygen atom is also shared with the central carbon atom, and the carbon atom shares one more electron with each oxygen atom:
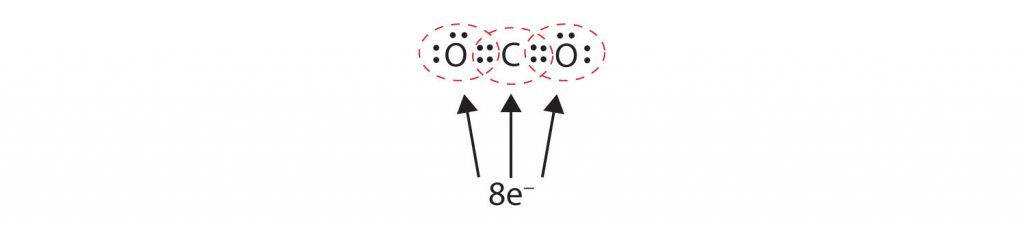
In this arrangement, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are now eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double dash:
Some molecules contain triple bonds, covalent bonds in which three pairs of electrons are shared by two atoms. A simple compound that has a triple bond is acetylene (C2H2), whose Lewis diagram is as follows:
A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. In the formation of a simple or ordinary covalent bond, each atom supplies one electron to the bond – but that does not have to be the case. In the case of a coordinate covalent bond, one atom supplies both of the electrons and the other atom does not supply any of the electrons. The following reaction between ammonia and hydrochloric acid demonstrates the formation of a coordinate covalent bond between ammonia and a hydrogren ion (proton).
If these colorless gases are allowed to mix, a thick white smoke of solid ammonium chloride is formed.